info@bilgergmbh.de
+49 (0)6051 916 69-51

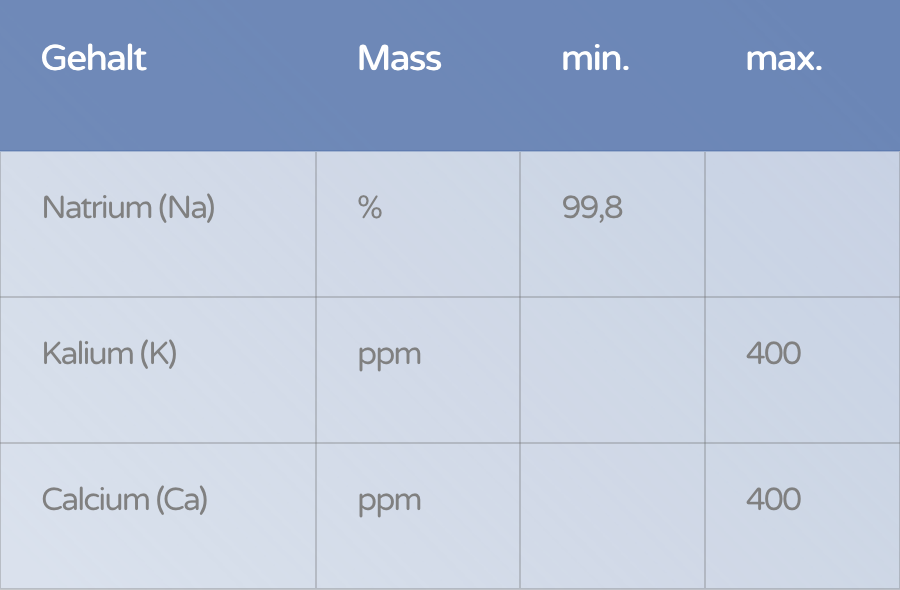
Content | Unit | min. | max. |
|---|---|---|---|
Sodium (Na) | % | 99,8 | |
Potassium (K) | ppm | 400 | |
Calcium (Ca) | ppm | 400 |
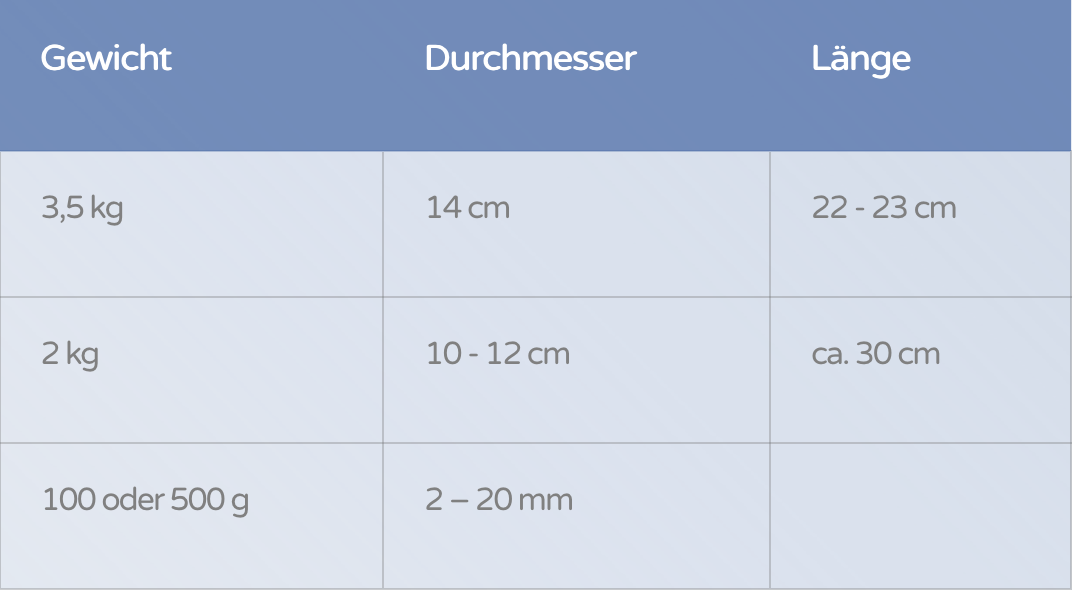
Weight | Diameter | Length |
|---|---|---|
3,5 kg | 14 cm | 22 - 23 cm |
2 kg | 10 - 12 cm | ca. 30 cm |

2 kg
3,5 kg
Lightly oiled cylindrical sodium bars in double-layer polyethylene bags in UN-tested steel drums with 140 kg or 150 kg net.

or in From our BINAL® vacuum-packed sodium in pieces from 25 g to 500 g
is very common on the earth's surface, especially in the form of sodium chloride. It is the seventh most abundant element and represents 2.83% of the Earth's crust mass.
Packaged sodium can be stored indefinitely if stored correctly. There must be no open water points or pipes carrying water ar prohibited in storage area.”. Sprinkler systems are not permitted.
See also:
Davy succeeded in producing metallic sodium for the first time in 1807 by electrolyzing moistened and melted caustic soda using a voltaic column. The first process on a technical scale was production using the Deville process in 1845.
Soda was heated with coal and lime in iron tubs to over 1000 °C and the sodium was obtained by condensing the steam. In 1891, Castner succeeded in developing an electrolytic production process for sodium from molten caustic soda.
This process led the way in sodium production for almost four decades. In 1921 the fusion electrolysis has been developed by Downs. Practically all sodium manufacturers use this process today. We use a mixture of NaCl/CaCl2/BaCl2, which melts at around 600 °C. The pure sodium obtained after filtration is then molded into bars or filled in containers.

Sir Humphry Davy
Gewerbepark Birkenhain 7a
63579 Freigericht
Tel: +49 (0)6051-91669-51
Fax: +49 (0)6051-91669-57
E-Mail: info@bilgergmbh.de
