info@bilgergmbh.de
+49 (0)6051 916 69-51

Sodium technology was originally used to clean oils containing PCBs. Since increased efforts have been made in recent decades to produce recycled diesel from plastic and other waste, there is another area of application for this technology. Recycled diesel has a proportion of sulfur-containing organic compounds that is above current limits and therefore cannot be used without desulfurization. Laboratory-scale experiments have proven that sodium technology is able to eliminate organosulfur compounds depending on the application temperature when treating the oil with sodium. However, this process is not yet used on an industrial or large-scale scale.
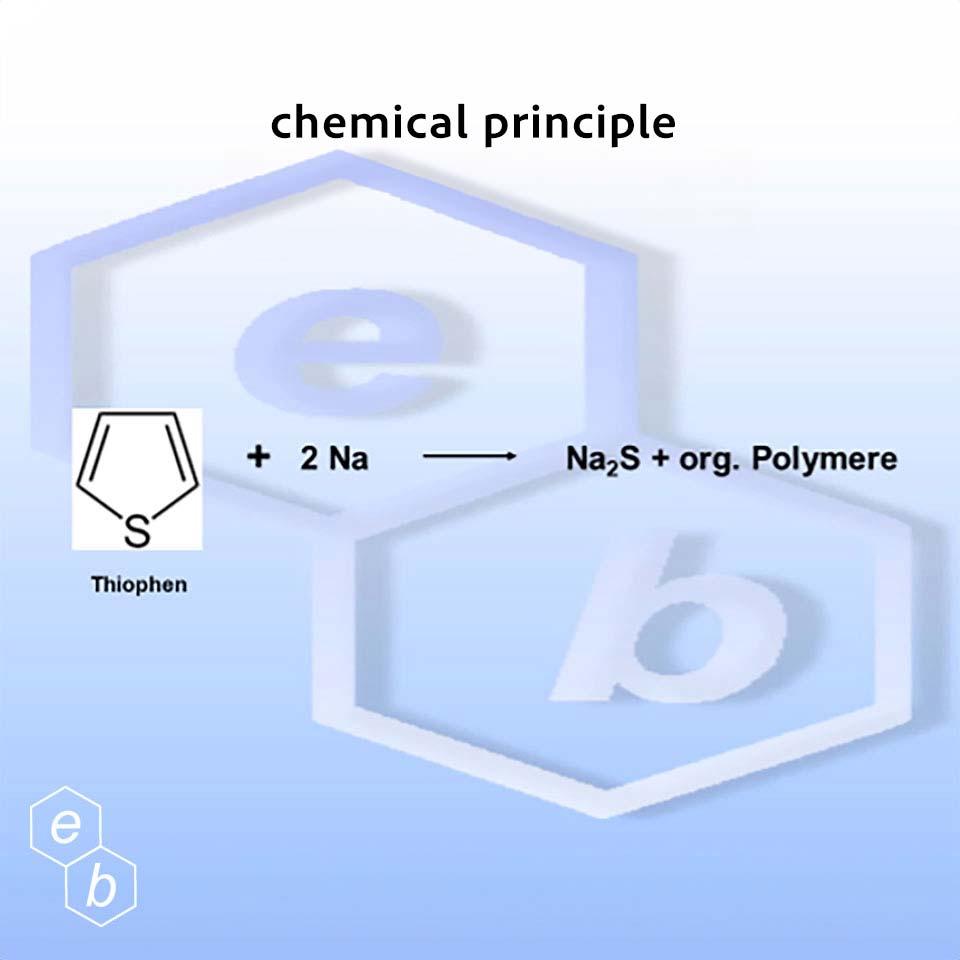
Elemental sodium reacts with the organically bound sulfur to form sodium sulfide, polymers and organic molecules. The reaction takes place at temperatures between 280 – 300 °C.
Examples of relevant sulfur-containing compounds:
All compounds of the three compound classes mentioned can be destroyed using sodium technology.
The required conditions for desulfurization vary greatly:
Since thiophenes make up a significant proportion of the sulfur compounds found in diesel fuels, a reaction temperature of around 300 °C must be selected in order to successfully carry out desulfurization using sodium technology.
Since the reaction rate of sodium is determined by the reaction surface available, the particle size of the individual sodium particles is of crucial importance. This correlation is shown in Fig. 2. Furthermore, the reaction rate is of course dependent on the temperature present.
A particle size of <10 µm should be achieved in order to achieve sufficient reactivity of the sodium.
Representation of the dependence of the reaction rate on the size of the surface of the sodium particles
Gewerbepark Birkenhain 7a
63579 Freigericht
Tel: +49 (0)6051-91669-51
Fax: +49 (0)6051-91669-57
E-Mail: info@bilgergmbh.de
